My group had the pleasure of contributing to the University’s open day. We had lots of interested future students learning about plasma, materials, and interdisciplinary research at the school of Agriculture, Food & Wine.



My group had the pleasure of contributing to the University’s open day. We had lots of interested future students learning about plasma, materials, and interdisciplinary research at the school of Agriculture, Food & Wine.


The National Food Waste Summit 2024 in Melbourne was a huge hit. There are so many great stories about reducing food waste coming out of the organisation from new research, to new policies, to behaviour change.
Here are two food waste superstar warriors from the ABC who contributed to our event: Craig Reucassel and Costa Georgiadis.


Great job Mel discussing your PhD research on the Food Waste Matters Podcast. Mel’s interview starts at 15.26
Journal cover artwork by Bryan Coad for Plasma Processes & Polymers special issue “Plasmas for Biointerfaces, volume 19, issue 10” featuring our review article “Plasma polymerization for biomedical applications“

The Australasian Society for Biomaterials and Tissue Engineering Conference (ASBTE) was held in Canberra from 18th to 20th April, 2017.
The conference venue was ANU commons. It was perfect autumn weather.

Conference venue: ANU commons
Thomas, Steph and Javad all presented posters
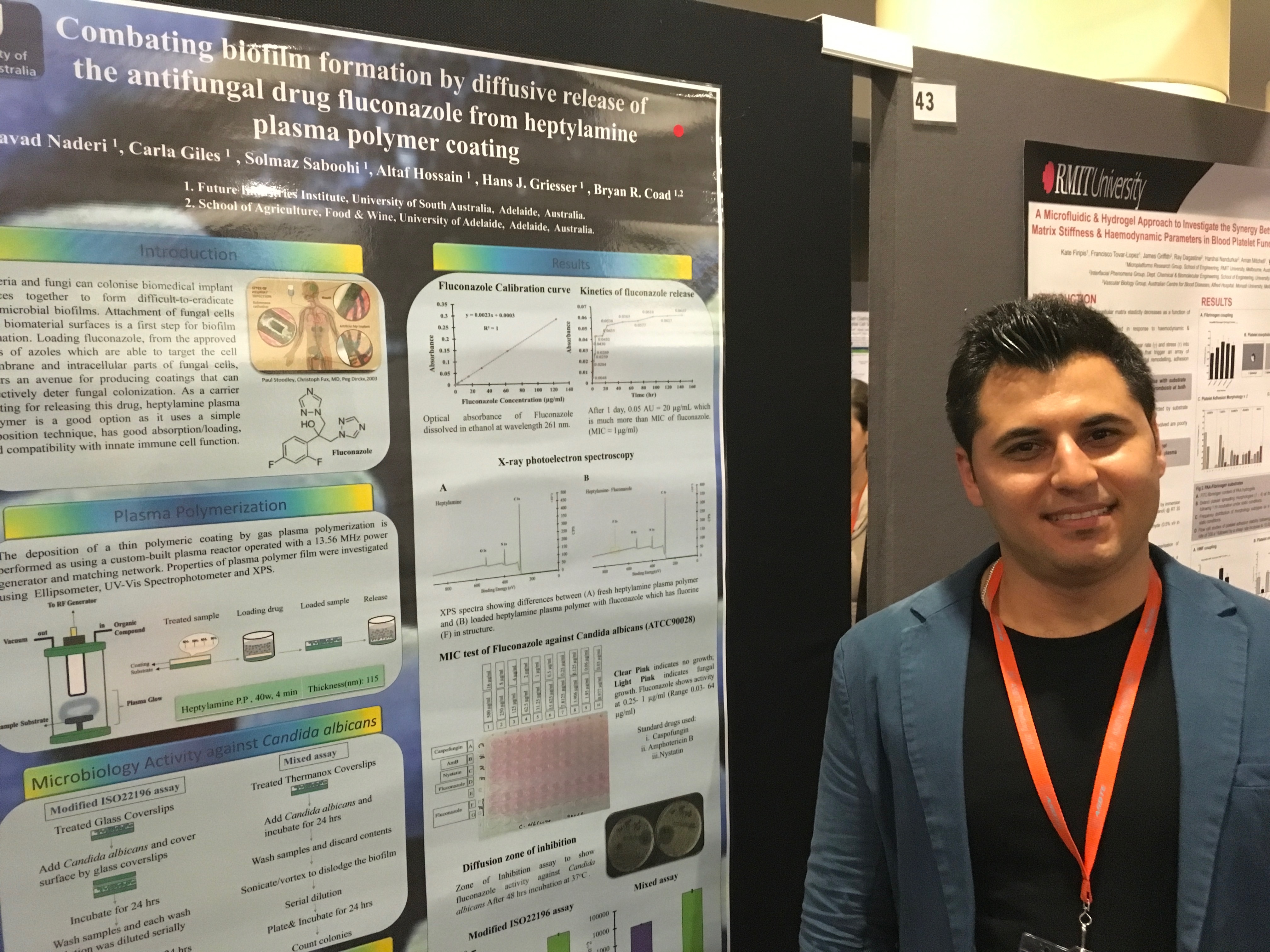
Javad presenting his poster

Steph presenting her poster for absentee Carla

Group photo: Javad, myself, Hans, Anouck, Thomas, and Steph
The conference dinner was filled with wonderful food, and a 25th anniversary slide show for past ASBTE conferences.

Hans was able to receive his Award of Excellence (officially awarded last year at the World Biomaterials Congress) and gave a moving speech about his years of research and its impact.

Hans Griesser receiving his ASBTE research excellence award
The conference was well attended right up to the last session.

ASBTE conference closing ceremony. Close to 200 delegates attended this years’ conference.
In the 20th century, polymers (plastics) were highly valued for their bulk properties – being lightweight and mouldable into any form. Their ubiquity has truly revolutionised the way in which we live our lives. In the 21st century, we will transform the way in which we use polymers from passive, bulk materials into thin film coatings that can be applied to virtually any underlying material. Thus, metals, ceramics, glass, even textiles and paper can be covered with a polymeric coating that will enhance their functional surface properties.

Understanding the plasma polymeristion process will help to innovate coatings on biointerfacing surfaces
One technology for making these polymeric coatings uses a state of matter known as plasma. It was previously thought that little control could be exerted over plasma polymerisations because the chaotic nature of this high-energy technique produced polymers with a scrambled structure. Led by Professor Hans Griesser, our new Australian Research Council Discovery Project titled “Order from Chaos” will help us understand how to bring order to the plasma polymerisation process and harness it to make more sophisticated polymer coatings with enhanced functionality.
Outcomes from this project will allow us to functionalise materials that will have wide use in every-day life: as coatings for textiles, medical sensors, automotive and aerospace industries. Particularly for life-sciences, plasma polymerisation is already used to improve the properties of diagnostic tools for biology (passive coatings), but our new understanding will allow more complicated surface coatings to be applied: e.g. biointerfacing surfaces as low-fouling surface coatings, or scaffolds on which to engineer tissues. It is because we will demonstrate how to add value and do more with the plasma polymerisation process that we see very good potential to help to develop Australia’s advanced manufacturing sector.
An Australian Research Council Discovery Project was awarded to Prof Hans Griesser, Prof Robert Short, Dr Bryan Coad, and Dr Andrew Michelmore for the years 2016 – 2018.

Surface coatings with covalently attached caspofungin are effective in eliminating fungal pathogens J. Mater. Chem. B, 2015, DOI: 10.1039/C5TB00961H. Click here.
We often think of pharmaceuticals as “little magic bullets” that circulate around the body once swallowed or injected. But targeting can be hit or miss for antimicrobial agents because sometimes the infection can be highly localized and associated with a particular material like when present on the surface of a catheter. Traditional drug administration has the “ammunition” circulating around the body to non-target areas and therefore, diluted greatly. Additionally, bacterial or fungal infections on surfaces are tough to eradicate from the outside-in because they “armoured” by a protective matrix of slime. The result is that the valuable ammunition goes largely wasted and the attack against the problem biofilm is often too weak to eliminate it. A very serious complication can arise from the development of resistance because bugs live by the adage that what does not kill them, makes them stronger.
For devices that are commonly colonised by harmful fungal invaders (such as catheters, breathing tubes, and hip and knee implants), it would be better to incorporate the drug onto the material with a surface coating, present at high concentrations, which could kill the very first bugs that try to attach and settle on the surface.
In this work, (published now in Journal of Materials Chemistry B) we asked the question whether a potent antifungal drug called caspofungin could still act as an effective antifungal agent by eliminating potential fungal colonisers after being irreversibly bound or tethered onto a surface. What is interesting for our research group, and the pharmaceutical industry, is to see whether or not drugs could be now formulated as part of a surface coating (a 2-dimensional surface administration) as compared to the traditional mechanism of action which is understood to be the freely circulating drug in 3-dimensions.
But how can one be sure that the drug is irreversibly attached to the surface? After all, drugs could be initially weakly bound to surfaces but then, desorb back into solution. Then we would then not be able to claim that this mechanism of action is any different to drugs administered systemically. So we turned this question on its head: we attempted to not only bind our target drug (caspofungin) to the surface, but we also tried two similar drugs that we knew wouldn’t be able to bind strongly. Surprisingly, what we found was that all three drugs initially had the ability to attach to the surface, albeit through weak forces that could be disrupted through soaking. Next we were able to investigate with rigorous washing procedures how we could remove all traces of weakly-adsorbed drugs from the surface, convincing us that only caspofungin, which exclusively possessed the ability to bind to the surface through permanent bonds, was covalently attached.
Armed with this knowledge, we challenged these caspofungin-bound surface coatings with four different Candida species which are common in infected medical devices. The caspofungin surface coatings were found to kill all of these species to some degree, with nearly complete elimination (98%) of one of the most prevalent fungal pathogens, Candida albicans.
Caspofungin has never before shown active killing against fungal pathogens when formulated as a surface coating and now represents a new way of thinking about this drug. This could influence the way medical devices are made or the thinking around traditional drug administration and dosing. The next step in our research will be to show precisely how this drug is able to disrupt and kill the cell when it settles on the surface by investigating its mechanism of action. We see this research as importantly contributing to a clinical need for new anti-infective materials and surface coatings — especially the need for therapies specifically targeting fungal pathogens and the use of antifungal drugs.
Published in Royal Society of Chemistry: Journal of Materials Chemistry B
B.R. Coad, S.J. Lamont-Friedrich, L. Gwynne, M. Jasieniak, S.S. Griesser, A. Traven, A.Y. Peleg and H.J. Griesser Surface coatings with covalently attached caspofungin are effective in eliminating fungal pathogens J. Mater. Chem. B, 2015, DOI: 10.1039/C5TB00961H
Dr. Bryan Coad is a Senior Research Fellow and Group Leader of the Mycology / Surface Interfaces Group at the Future Industries Institute at the University of South Australia. He possesses over 15 years’ experience in the development of biomaterials and bioactive surface coatings. His research is aligned with clinical need for novel strategies for using antibiotic compounds reducing the development of antimicrobial resistance. He is developing these strategies with support from the Australian Government through competitive granting schemes and is bringing innovative medical devices to market by actively engaging with industry.
Starting 31st August, the Mawson Institute, The Ian Wark Research Institute, and the Centre for Envrionmental Risk Assessment and Remediation (CERAR) will no longer exist as separate entities.
The university has merged these three institutes into the Future Industries Institute (FII).
For my research group, not much will initially change as my position as Senior Research Fellow will continue on in the new institute.
We’re looking forward to the new opportunities that will come in the Future.
Uniform plasma treatment of micron-sized particles can be challenging because the particles need to be suspended in the plasma discharge. What does this have to do with a common stereo speaker playing music or a rotating apparatus commonly used for purifying organic chemical compounds?
Shake, rattle & roll.

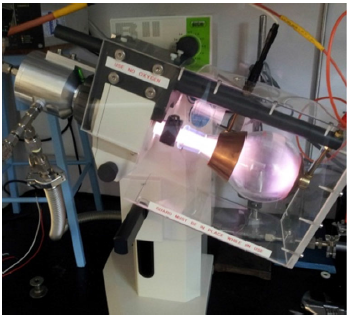
Putting the particles on top of a speaker playing music shakes and rattles the particles in the plasma discharge and allows them to be evenly coated.
Or, tumbling the particles in a rotating flask containing the plasma discharge rolls them around and gives them an even coating.
These are the two ideas behind our new publication from Wark and Mawson Institute researchers in Plasma Processes and Polymers:
Common laboratory or household equipment can be readily adapted to plasma discharge apparatus creating a low-cost, yet effective way to solve the challenge of depositing plasma coatings on small particles.
The article has just been published online. You can read more about it here.
Hospital acquired infections are a public health crisis and a leading cause of death world-wide. In the USA over 200 people die each day from hospital acquired infections with over 80% of these related to contaminated surfaces of implanted medical devices. While there is a good level of understanding and research into bacterial infections, fungal infections are the hidden killers – often missed in diagnosis. Treatment with antifungal drugs is therefore delayed and often comes too late, with devastating consequences. Rapidly multiplying on surfaces in a protected slimy coating known as a biofilm, mature fungal infections can spread into the blood and are often more effective when compared to bacteria in host mortality — giving a less than 50/50 chance of patient survival.
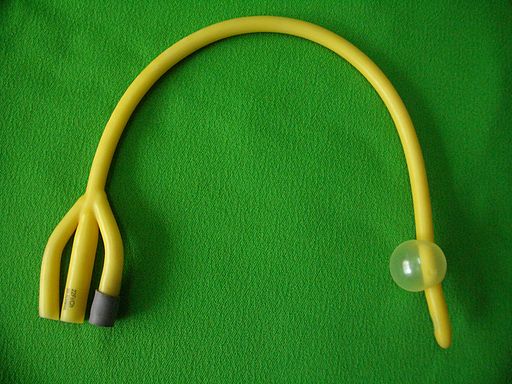
The surface of urinary catheters is frequently a site for bacterial and fungal biofilm growth and often leads to infection.
The understanding of how to fight fungal infections from surfaces will be given a major boost by a 4-year grant from the Australian Research Council’s Discovery Grant Program. Recognizing the need for cross disciplinary research at the nexus of physical chemistry, materials science, and biology, the team led by University of South Australia Researchers (the Mycology / Surface Interfaces Group) will bring together world-leading polymer chemists and mycologists across three countries (Australia, Switzerland and the UK) to drive breakthrough research into effective antifungal surface coatings.
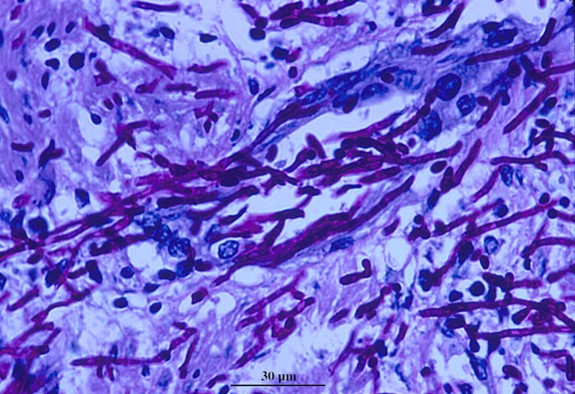
Candida albicans, common and mostly benign to healthy individuals, becomes deadly inside the body when it forms biofilms and invades tissues.
Professor Hans Griesser and Senior Research Fellow Dr. Bryan Coad of UniSA’s Mawson Institute will lead the grant. “This grant will bring Australian discoveries to the forefront in what is a new research area,” says Dr. Coad. “Strengthened by the expertise from our international partners, we see potential to deliver outcomes likely to resonate with our scientific peers the world over.”
The four-year research program will develop advanced material surface coatings at UniSA and unravel the design principles needed to transform them into 3-D polymeric platforms in collaboration with partner investigator Prof. Harm-Anton Klok at EPFL Switzerland, an expert in bioactive polymer coatings. Direct visualization of fungal cells on surfaces using live cell imaging by partner investigator Prof. Nick Read at the University of Manchester (Manchester Fungal Infection Group) will reveal the fate of cells and provide direct evidence for the mechanisms of action. “The team will tie the physical and chemical properties of their surface coatings to their specific biological consequences. For fungi, this has never been done before so there is fantastic opportunity to make fundamental discoveries,” says Dr. Coad.