Surface coatings with covalently attached caspofungin are effective in eliminating fungal pathogens J. Mater. Chem. B, 2015, DOI: 10.1039/C5TB00961H. Click here.
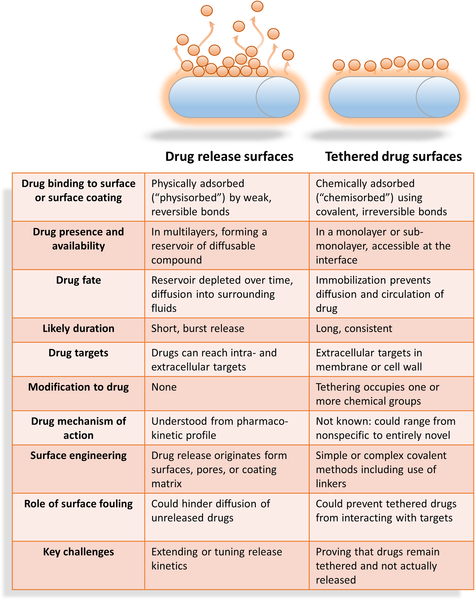
We often think of pharmaceuticals as “little magic bullets” that circulate around the body once swallowed or injected. But targeting can be hit or miss for antimicrobial agents because sometimes the infection can be highly localized and associated with a particular material like when present on the surface of a catheter. Traditional drug administration has the “ammunition” circulating around the body to non-target areas and therefore, diluted greatly. Additionally, bacterial or fungal infections on surfaces are tough to eradicate from the outside-in because they “armoured” by a protective matrix of slime. The result is that the valuable ammunition goes largely wasted and the attack against the problem biofilm is often too weak to eliminate it. A very serious complication can arise from the development of resistance because bugs live by the adage that what does not kill them, makes them stronger.
For devices that are commonly colonised by harmful fungal invaders (such as catheters, breathing tubes, and hip and knee implants), it would be better to incorporate the drug onto the material with a surface coating, present at high concentrations, which could kill the very first bugs that try to attach and settle on the surface.
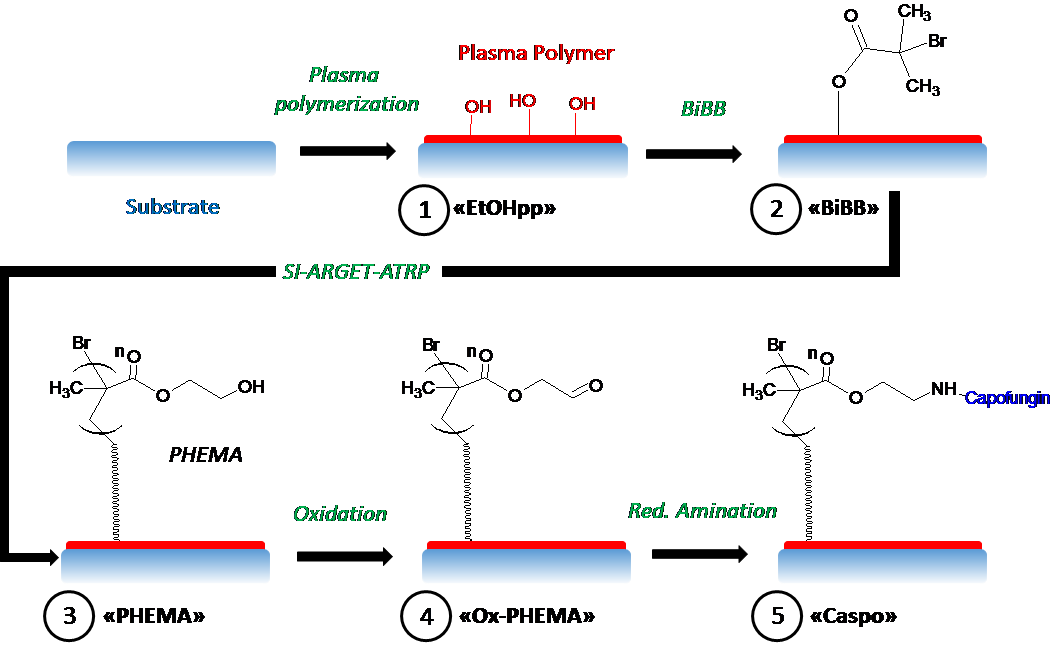
In this work, (published now in Journal of Materials Chemistry B) we asked the question whether a potent antifungal drug called caspofungin could still act as an effective antifungal agent by eliminating potential fungal colonisers after being irreversibly bound or tethered onto a surface. What is interesting for our research group, and the pharmaceutical industry, is to see whether or not drugs could be now formulated as part of a surface coating (a 2-dimensional surface administration) as compared to the traditional mechanism of action which is understood to be the freely circulating drug in 3-dimensions.
But how can one be sure that the drug is irreversibly attached to the surface? After all, drugs could be initially weakly bound to surfaces but then, desorb back into solution. Then we would then not be able to claim that this mechanism of action is any different to drugs administered systemically. So we turned this question on its head: we attempted to not only bind our target drug (caspofungin) to the surface, but we also tried two similar drugs that we knew wouldn’t be able to bind strongly. Surprisingly, what we found was that all three drugs initially had the ability to attach to the surface, albeit through weak forces that could be disrupted through soaking. Next we were able to investigate with rigorous washing procedures how we could remove all traces of weakly-adsorbed drugs from the surface, convincing us that only caspofungin, which exclusively possessed the ability to bind to the surface through permanent bonds, was covalently attached.
Armed with this knowledge, we challenged these caspofungin-bound surface coatings with four different Candida species which are common in infected medical devices. The caspofungin surface coatings were found to kill all of these species to some degree, with nearly complete elimination (98%) of one of the most prevalent fungal pathogens, Candida albicans.
Caspofungin has never before shown active killing against fungal pathogens when formulated as a surface coating and now represents a new way of thinking about this drug. This could influence the way medical devices are made or the thinking around traditional drug administration and dosing. The next step in our research will be to show precisely how this drug is able to disrupt and kill the cell when it settles on the surface by investigating its mechanism of action. We see this research as importantly contributing to a clinical need for new anti-infective materials and surface coatings — especially the need for therapies specifically targeting fungal pathogens and the use of antifungal drugs.
Published in Royal Society of Chemistry: Journal of Materials Chemistry B
B.R. Coad, S.J. Lamont-Friedrich, L. Gwynne, M. Jasieniak, S.S. Griesser, A. Traven, A.Y. Peleg and H.J. Griesser Surface coatings with covalently attached caspofungin are effective in eliminating fungal pathogens J. Mater. Chem. B, 2015, DOI: 10.1039/C5TB00961H
Dr. Bryan Coad is a Senior Research Fellow and Group Leader of the Mycology / Surface Interfaces Group at the Future Industries Institute at the University of South Australia. He possesses over 15 years’ experience in the development of biomaterials and bioactive surface coatings. His research is aligned with clinical need for novel strategies for using antibiotic compounds reducing the development of antimicrobial resistance. He is developing these strategies with support from the Australian Government through competitive granting schemes and is bringing innovative medical devices to market by actively engaging with industry.